Page 13 - 南京医科大学学报自然科学版
P. 13
第41卷第6期 袁 逸,戴程婷,李一卉,等. 大宫内发育迟缓新生小鼠胰腺发育转录组分析[J].
2021年6月 南京医科大学学报(自然科学版),2021,41(06):785-795 ·791 ·
A B
Systemic lupus erythematosus 1 230 up
Alcoholism 984 down
Transoriptional misregulation in cancers
Viral carcinogenesis Num of Genes 738
Cell cycle
Spliceosome GeneNumber 492
10
p53 signaling pathway 246
20
DNA replication 30
FoxO signaling pathway 40 0 behavior cell aggregation cell killing cellular process detoxification growth localization locomotion reproduction rhythmic process signaling cell junction cell cell part membrane membrane part nucleoid organelle organelle part other organism synapse synapse part virion virion part catalytic activity catalytic activity protein tag
transcription factor activity, protein binding
binding
extracellular matrix component
nucleic acid binding transcnption factor activity
molecular function regulater
multiceilular organismal process
cellular component organization of biogenesis
metabolic process
multi⁃organism process
Pathway Dorso⁃ventral axis formation QValue biological adhesion biological regulation developmental process immune system process presynaptic process invoived in synaptic transmission reproductive process response to stimulus single⁃organism process extracellular matrix extracellular region extracellular region part macromolecular complex membrane⁃enclosed lumen other organism part supramolecular fiber chemoatlractart activity electron carrier activity molecular
50
MicroRNAs in cancer
Malaria
0.075
mRNA surveillance pathway
0.050
Proteoglycans in cancer
0.025 Biological Process Cellular Component Molecular Function
Steroid biosynthesis
Cytokine⁃cytokine receptor interaction
Hematopoietic cell lineage
Central carbon metabolism in cancer
Oocyte meiosis
PI3K⁃Akt signaling pathway
0.05 0.10 0.15 0.20
Rich Factor
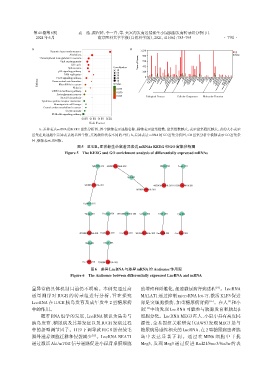
A:差异表达mRNA的KEEG富集分析图,图中纵轴表示通路名称,横轴表示富集指数,富集指数越大,表示富集程度越大,点的大小表示
富集在此通路中差异表达的基因个数,点的颜色代表不同的P值;B:差异表达mRNA的GO富集分析图,GO富集分析中横轴表示GO富集条
目,纵轴表示基因数。
图5 IUGR、正常新生小鼠差异表达mRNAs KEEG和GO富集分析图
Figure 5 The KEEG and GO enrichment analysis of differentially expressed mRNAs
Epb41l3⁃216 D030028A08Rik⁃203 Nfkb2⁃204 Acss1⁃201
Sp2⁃203
2410021H03Rik⁃201 4833438C02Rik⁃201 E130215H24Rik⁃201
D030028A08Rik⁃201
Epb41l3⁃215
Tiparp⁃201 Rap1gap⁃201 4931406P16Rik⁃202 Colca2⁃202 Tcf21⁃201 Fyn⁃206
4931440P22Rik⁃201 Rap1gapos⁃201 Gm12758⁃201 2010007H06Rik⁃202 Gm10824⁃201 Gm16364⁃205
Sun2⁃201 Abca3⁃203
Gm16575⁃201 D330041H03Rik⁃205
图6 差异LncRNA与差异mRNA的 Antisense作用图
Figure 6 The Antisense between differentially expressed LncRNA and mRNA
量异常的具体机制目前仍不明确。本研究通过高 的增殖和纤维化,加速糖尿病肾病进程 。LncRNA
[15]
通量测序对 IUGR 的转录组进行分析,旨在探究 MALAT1 通过抑制 microRNA let⁃7f、激活 KLF5 促进
LncRNA 在 IUGR 胰岛发育及成年发生 2 型糖尿病 肾足突细胞损伤,加重糖尿病肾病 [16] 。在人 和小
[9]
中的作用。 鼠 [10] 中均发现 LncRNA 可能参与胰腺发育和胰岛β
随着 RNA 组学的发展,LncRNA 被认为是参与 细胞分化。LncRNA MEG3在人、小鼠中具有高度同
胰岛发育、糖尿病及其并发症以及 IUGR 发病过程 源性,全基因组关联研究(GAWS)发现 MEG3 是与
中的新型调节因子。H19 下调导致 IUGR 胎盘绒毛 糖尿病易感性相关的LncRNA,在2型糖尿病患者胰
[12]
膜外滋养细胞迁移和侵袭减少 。LncRNA NEAT1 岛中表达显著下调。通过在 MIN6 细胞中干扰
通过激活Akt/mTOR 信号通路促进小鼠肾系膜细胞 Meg3,发现 Meg3 通过促进 Rad21/Smc3/Sin3α的表