Page 93 - 南京医科大学自然版
P. 93
第44卷第4期 喻 悦,王瑜亮,张 晓. 葡萄糖代谢重编程在胰腺癌耐药中的研究进展[J].
2024年4月 南京医科大学学报(自然科学版),2024,44(04):524-535,572 ·531 ·
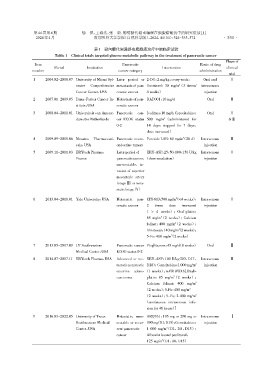
表1 靶向糖代谢通路在胰腺癌治疗中的临床试验
Table 1 Clinical trials targeted glucose metabolic pathway in the treatment of pancreatic cancer
Phase of
Item Pancreatic Route of drug
Period Institution Intervention clinical
number cancer category administration
trial
1 2004.02-2008.07 University of Miami Syl⁃ Later period or 2⁃DG:2 mg/kg,every week; Oral and Ⅰ
2
vester Comprehensive metastasis of pan⁃ Docetaxel:30 mg/m(3 times/ intravenous
Cancer Center,USA creatic cancer 4 weeks) injection
2 2007.01-2009.05 Dana⁃Farber Cancer In⁃ Metastasis of pan⁃ RAD001:10 mg/d Oral Ⅱ
stitute,USA creatic cancer
3 2008.04-2011.01 Universiteit van Amster⁃ Pancreatic can⁃ Ivolimus:10 mg/d;Capecitabine: Oral Ⅰ
2
dam,the Netherlands cer ECOG status 500 mg/m (administered for &Ⅱ
0⁃2 14 days,stopped for 7 days,
dose increased)
2
4 2009.09-2015.06 Novartis Pharmaceuti⁃ Pancreatic neuro⁃ Paretide LAR:60 mg/m(28 d) Intravenous Ⅱ
cals,USA endocrine tumors injection
5 2009.11-2011.03 ERYtech Pharma, Later period of ERY⁃ASP:25,50,100,150 U/kg Intravenous Ⅰ
France pancreaticcancer,(dose escalation) injection
unresectable,in⁃
vasion of superior
mesenteric artery
(stageⅢ)or meta⁃
static(stage Ⅳ)
2
6 2013.04-2016.01 Yale University,USA Metastatic pan⁃ CPI⁃613:500 mg/m(≤4 weeks), Intravenous Ⅰ
creatic cancer 2 times dose increased injection
( > 4 weeks);Oxaliplatin:
2
65 mg/m(2 weeks);Calcium
folinat:400 mg/m(2 weeks);
2
Irinotecan:140 mg/m(2 weeks);
2
5⁃Fu:400 mg/m(2 weeks)
2
7 2013.05-2017.09 UT Southwestern Pancreatic cancer Pioglitazone:45 mg/d(8 weeks) Oral Ⅱ
Medical Center,USA ECOG status 0⁃2
8 2014.07-2017.11 ERYtech Pharma,USA Advanced or me⁃ ERY⁃ASP:100 U/kg(D3,D17, Intravenous Ⅱ
tastaticpancreatic D28);Gemcitabine:1 000 mg/m 2 injection
exocrine adeno⁃ (1 weeks);mFOLFOX6[Oxali⁃
2
carcinoma platin:85 mg/m(2 weeks);
Calcium folinat: 400 mg/m 2
(2 weeks);5⁃Fu:400 mg/m 2
(2 weeks);5⁃Fu:2 400 mg/m 2
(continuous intravenous infu⁃
sion for 46 hours)]
9 2016.03-2022.05 University of Texas Metastatic,unre⁃ ARQ761:195 mg or 290 mg or Intravenous Ⅰ
Southwestern Medical sectable or recur⁃ 390 mg(D1,D15);Gemcitabine: injection
2
Center,USA rent pancreatic 1 000 mg/m(D1,D8,D15);
cancer Albumin bound paclitaxel:
125 mg/m(D1,D8,D15)
2